A revolutionary approach in brain retraction
Market and Clinical Need for VBAS
Since the introduction of the first operative microscope 50+ years ago, microsurgery (and more recently, endoscopic surgery) has become an indispensable technique in neurosurgery. In any surgical procedure adequate visualization of the operative field is critical. The standard of care is traditionally ribbon or blade retractors used to create and maintain visual corridors to access targets within the brain. The brain, like other sensitive tissue, is subject to injury from retraction – most evident but not limited to approaches to deep-seated intracranial lesions.
VBAS was designed to address this market need and has a substantial body of clinical data validating its design attributes and clinical benefits.
Superior Shape

- The VBAS tubular shape disperses retraction forces over a greater surface area and has no edges where pressure build up is most common.
- Blunt tip allows for progressive dilation that permits the splitting of white matter rather than its transection.
- Lower risk of ischemic complications and results in faster wound healing and shorter patient recovery time.
- Clinical data demonstrates usage of VBAS leads to lower morbidity and shorter hospital stay. This supports anecdotal comments that the VBAS also significantly reduces tissue disruption and blood loss, resulting in shorter operating times.
Superior Field of View
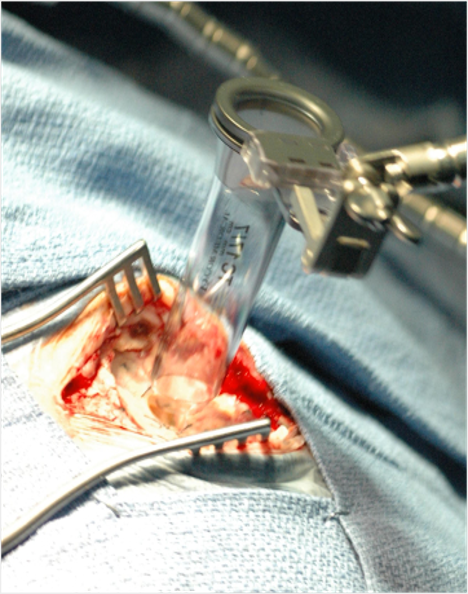
- VBAS is made of transparent polycarbonate, which significantly enhances visibility in the surgical field. Unlike metal retractors that can block the surgeon’s view or create glare under operating theater lights, the clear walls of VBAS allow for continuous monitoring of surrounding tissue during the approach and procedure.
- VBAS is coated in biocompatible non-reflective ink which minimizes the reflection issues experienced with other retractors.

Improved Working Channel
- Its elliptical shape further improves the operating environment by providing an increased working space without requiring a larger incision and allowing bifocal vision. Surgeons can insert two instruments at once and perform bimanual surgery, a technique that allows for greater control and precision. The enclosed channel not only creates more room but also protects peripheral tissue from accidental damage caused by surgical tools or heat.
- Provides an air instead of CSF medium that delivers better intra-operative visualization. VBAS provides protection of peripheral anatomy from inadvertent instrumental or thermal damage
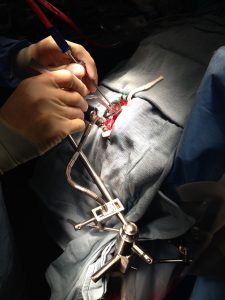
Compatible with Neuronavigation

Real-World Impact of VBAS
The real-world impact of VBAS has been demonstrated in numerous challenging cases. For example, a patient at Ohio State University was taking Avastin®, a drug that slows wound healing, which made her ineligible for standard surgery. With VBAS, the surgeon was able to access the lesion through a 3-centimeter incision, complete the procedure, and discharge the patient without complications. In another case at Lincoln Medical Center in New York, a bullet fragment lodged in the brain was successfully removed—an operation the chief neurosurgeon said would not have been attempted without VBAS.
VBAS is more than a novel device; it is backed by a growing body of clinical evidence with 50 peer-reviewed studies and numerous additional clinical papers published involving over 580 patients. These studies report consistently positive outcomes, including shorter operating times, reduced brain damage, and faster hospital discharge. These advantages not only improve patient safety but also reduce overall treatment costs for hospitals.
VBAS has been approved and used in over 350 hospitals in the United States and numerous countries internationally, including major markets such as Japan, Canada, the United Kingdom, and countries in the European Union. Vycor supports this footprint through a network of over 1000 U.S. independent neuro-surgical sales representatives and international distributors specializing in neurosurgical devices. Over 45,000 neurosurgical procedures have been carried worldwide out utilizing VBAS.
Looking ahead, one of the most promising areas for further VBAS adoption is in the treatment of intracerebral hemorrhage (ICH)—a condition in which bleeding occurs within the brain. This condition often requires precise access to remove the blood clot and relieve pressure. With increased focus from the clinical community on surgical intervention for ICH and using minimally invasive tubular retractors for ICH evacuation, VBAS is well-positioned to support this evolving standard of care.
Together, the device’s design innovations, clinical support, and surgeon feedback point to its growing role in modern neurosurgery. Vycor continues to improve the system, refine its applications, and expand its use globally along with the development of new variants.
Different from the Rest
Fully disposable and metal-free: Maximizes hospital efficiency, no sterilization cycles and risk of retained bioburden.
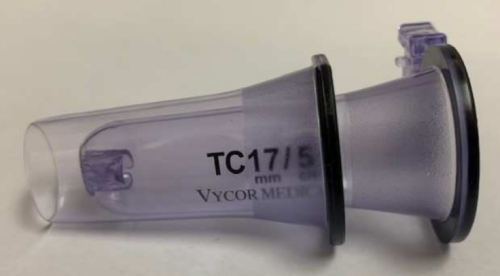
Optically clear plastic: Significant differentiator, gives surgeons direct visualization of surrounding tissue through retractor walls during navigation to target.
Elliptical (oval) shape: Compared to circular tubes the oval profile of VBAS offers more lateral working space—facilitating bimanual work.
Reduced pressure: The small holes at the base of the introducer allow cerebral fluid to escape during insertion, reducing intracranial pressure spikes and potentially minimizing tissue trauma; particularly important when inserting a tubular retractor into a closed system such as the intracranial ventricular.
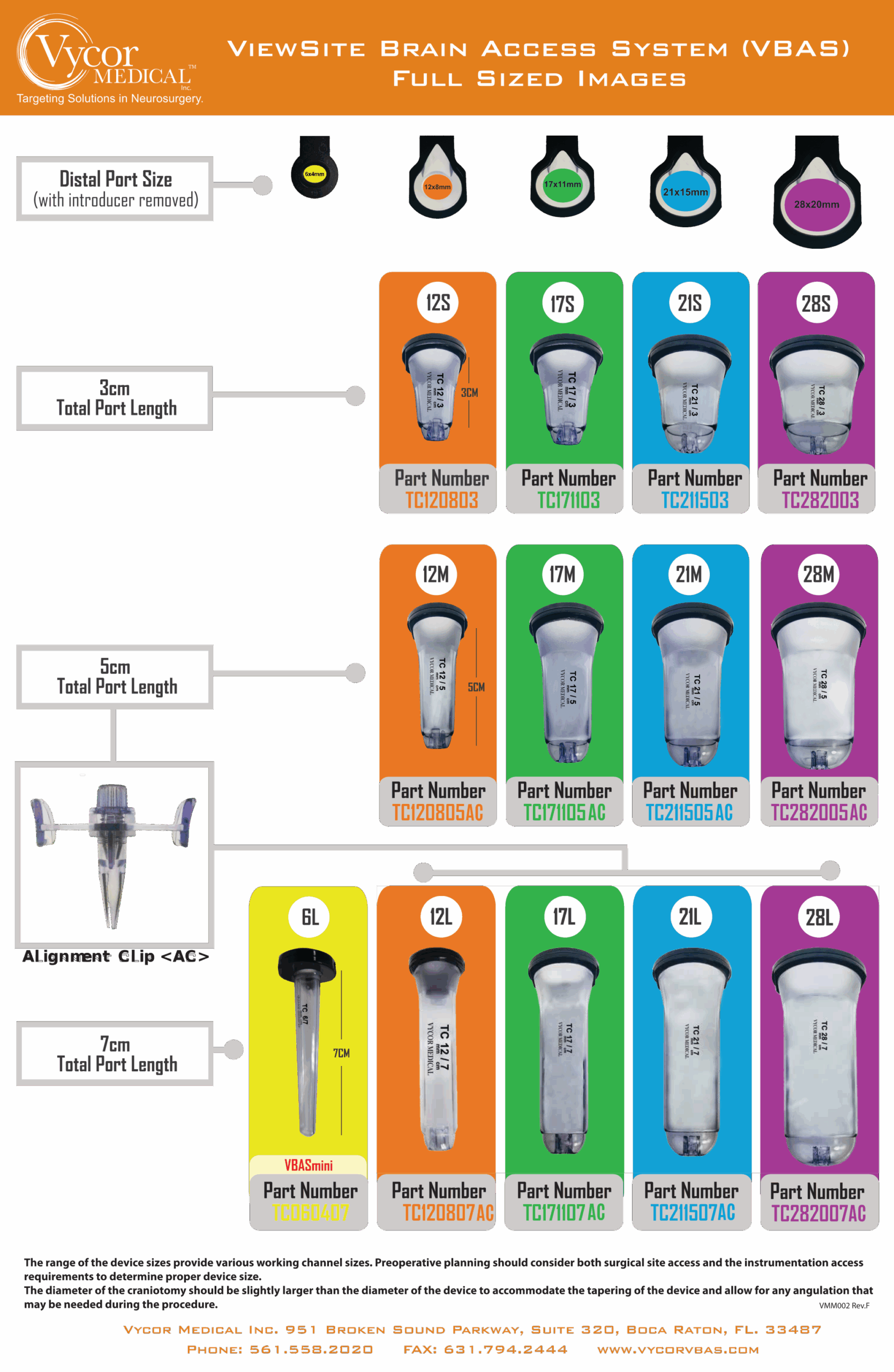
Multiple sizes: VBAS is offered in a range of sizes, from 6mm to 28mm width and 3cm to 7cm length, offering full flexibility for a broad range of procedures. Hospitals can purchase only the sizes they need, no requirement for ‘bundle’ purchasing.
Product Descriptions